Biomed Industries, Inc. Advances First-of-Its-Kind Oral NA-931 and Semaglutide Combination Therapy for Treating Obesity
Biomed Industries, Inc. Advances First-of-Its-Kind Oral NA-931 (Bioglutide) and Injectable Semaglutide Combination Therapy for the Treatment of Obesity
Phase 2 data show NA-931 has meaningful efficacy and excellent safety/tolerability profile. Combination therapy of NA-931 and semaglutide may further support weight loss in adults with severe obesity.”
SAN JOSE, CA, UNITED STATES, August 20, 2025 /EINPresswire.com/ -- SAN JOSE, Calif., Aug. 20, 2025 — Biomed Industries, Inc. (“Biomed”) today announced it is in active discussions with several pharmaceutical manufacturing and distribution partners to commercialize its novel, oral obesity candidate, NA-931 (Bioglutide™), both as a standalone therapy and in combination with semaglutide. — Dr. Lloyd L. Tran, CEO of Biomed
Subject to regulatory pathways and local intellectual property considerations, Biomed plans to pursue combination commercialization in markets where certain semaglutide patent protections are expected to expire in early 2026, including Canada, China, India, and Brazil.
NA-931 (Bioglutide™)—an investigational, oral quadruple-receptor agonist—recently completed a Phase 2 clinical trial demonstrating clinically meaningful weight loss with fewer and milder adverse events compared with currently available single-, dual-, or triple-agonist therapies, many of which are injectable. Phase 2 results of NA-931 were presented at the 85th Scientific Sessions of the American Diabetes Association (ADA), June 19–23, 2025 in Chicago, and at the Annual Meeting of Endocrinology (ENDO), July 12–15, 2025 in San Francisco.
Preclinical studies combining Bioglutide with semaglutide or tirzepatide showed synergistic effects, including enhanced weight loss and improvements in metabolic biomarkers associated with obesity and its complications. These monotherapy and combination findings will be further evaluated in Biomed’s Phase 3 clinical program, expected to begin in the coming months.
In preparation for commercialization, the company plans to launch NA-931 (Bioglutide™) as monotherapy and, in select geographies where semaglutide patent protection is expected to expire in 2026, as a combination therapy with ultra-low-dose semaglutide under the proposed brand Biosemtide™.
“Our Phase 2 data suggest that NA-931 demonstrates meaningful efficacy with a favorable safety and tolerability profile,” said Dr. Lloyd Tran, CEO of Biomed. “Preclinical studies indicate that co-administration with semaglutide may further improve weight loss. By potentially allowing lower doses of semaglutide in combination with NA-931, our goal is to help adults with severe obesity achieve weight loss more quickly while maintaining a strong safety profile. We believe this approach could provide a more accessible and cost-effective option for patients.”
THE URGENT NEED FOR SAFE, EFFECTIVE, AND AFFORDABLE OBESITY TREATMENTS
Obesity is a critical global health challenge linked to serious comorbidities, including type 2 diabetes, cardiovascular disease, liver disease, and chronic kidney disease. More than 650 million people worldwide live with obesity, and prevalence is projected to rise substantially by 2035. Current anti-obesity medicines such as semaglutide and tirzepatide are injectable drugs typically priced around $1,000 per month for many patients in the U.S., placing them out of reach for those without insurance coverage for obesity care. Biomed intends to price oral NA-931 to enable broader global access.
ABOUT BIOMED INDUSTRIES, INC.
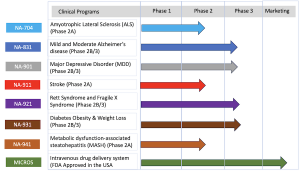
Biomed Industries, Inc. is a pioneering biopharmaceutical company developing and commercializing transformative therapies for chronic and complex conditions. The company’s pipeline includes investigational treatments for Alzheimer’s disease, major depressive disorder (MDD), obesity, diabetes, metabolic dysfunction–associated steatohepatitis (MASH), stroke, alcohol use disorder, and rare diseases such as Rett syndrome. For more information, visit www.biomedind.com.
Media contact:
Michael Willis
Biomed Industries, Inc.
+1 800-824-5135
email us here
Visit us on social media:
LinkedIn
X
Biomed introduction video
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.